- myLubrizol
-
-
Search Pharmaceuticals
Lubrizol’s Apinovex™ polymers are high molecular weight polyacrylic acid excipients designed to provide both processing and formulation benefits for spray-dried amorphous solid dispersions (ASDs).
Apinovex polymers enable formulators to enhance the solubility of BCS Class II and IV APIs and develop more efficient oral dosage forms with high, stable drug loading.


Apinovex™ polymers can be easily incorporated into your drug project via spray drying or other solvent-based processes. This novel excipient has a broad API and solvent compatibility to produce Amorphous Solid Dispersions (ASD) of your brick-dust API.
| Test | Results |
|---|---|
| Acute oral toxicity | Not acutely toxic (LD50>5000 mg/kg) |
| Mutagenicity in bacteria (AMES) | Non-mutagenic |
| 28-day oral repeat dose study | Testing underway |
Apinovex chemistry is similar to LLS Health’s IID-listed Carbopol® polymers, which have been used in oral drug products for decades.
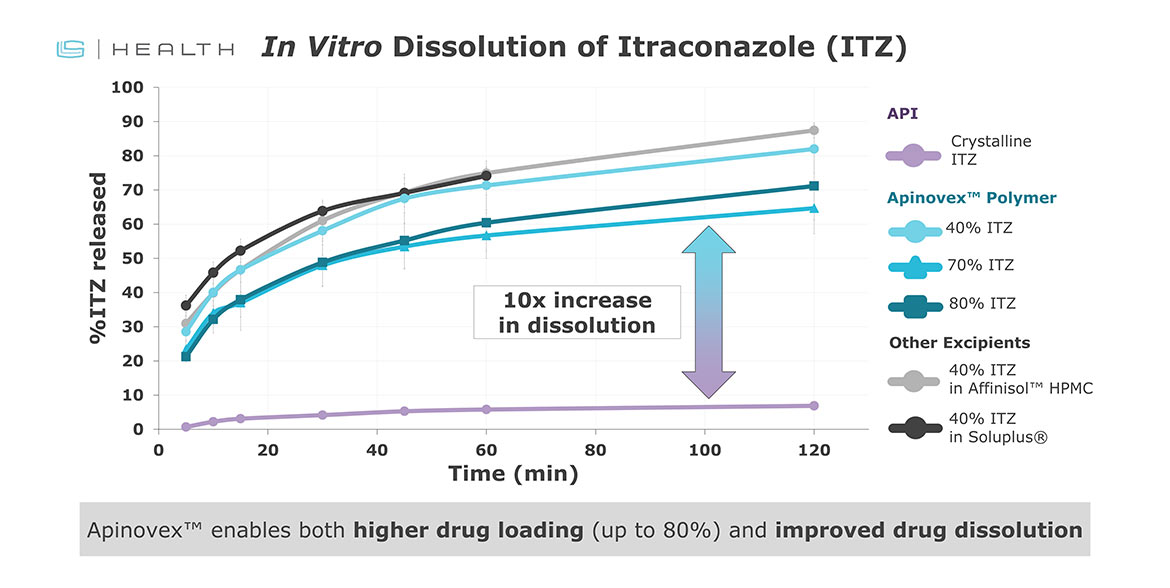
In case studies with itraconazole and ritonavir (BCS Class II drugs), Apinovex enabled:



Discover how our excipient can solve solubility issues for your most challenging oral drug projects.
Request a Sample