It’s no secret that solubility and bioavailability issues continue to challenge formulators and drug developers. Approximately 60-90% of potential new active pharmaceutical ingredients (APIs) in development pipelines, and more than 40% of those in reformulation, are poorly water-soluble. (1) Due to the increasing number of poorly water-soluble drug candidates, it is clear that effective solubility-enhancing solutions are needed. However, the statistics clearly demonstrate that there are still not enough effective technologies or excipients on the market – nor those which are saleable enough – to provide sufficient solubility enhancement.
The lack of appropriate solutions for scalable and efficient solubility enhancement is impacting development across many dosage forms. Of these, it’s important to highlight parenteral dosages due to the wide-ranging application of parenteral systems in various therapeutic areas. In 2019, the FDA estimated that around 42% of new molecular entities (NMEs) approved were parenteral formulations. (2) The wide-ranging applications of parenteral dosage forms mean that solubility challenges can impact a variety of therapeutic areas, including anti-cancer drugs.
Parenteral dosage forms are a key delivery system for the oncology sector. In 2017, 27.5% of parenteral NMEs were for oncology drugs. (3) For the industry to meet the continuous need for new and effective anticancer drugs, scalable solutions for enhancing parenteral solubility will be vital. Let’s take a closer look at oncology development to understand the specific needs of anti-cancer formulations and the tools and techniques available to improve solubility.
Understanding the current challenges for oncology drug formulation
In addition to the rising number of hydrophobic APIs in development, drug developers seeking viable oncology therapies must also mitigate the challenges of potent APIs and potential adverse effects. Compounds and delivery vehicles used to treat cancer are much more toxic than most other drug moieties used to treat other diseases.
Safety, quality, and efficacy are the core values driving the modern drug pipeline. To meet these pillars, early-stage discovery and development teams estimate and work towards the maximum tolerated dose (MTD), which is the highest dose that does not cause unacceptable side effects. However, increasingly toxic and insoluble drug candidates are adding to the complexities of striking the right balance.
If carefully considered, drug delivery methods can provide a tool to overcome these challenges. Ideally, oncology therapeutics would be delivered orally, as this negates the need for specialized equipment – such as a syringe and intravenous (IV) infusion apparatus – and it is also more appealing for patients. However, most APIs used in oncology treatments have poor oral bioavailability and have an increased potential for gastrointestinal (GI) tract irritation. Additionally, the overall toxicity of the oncology drug can be exacerbated by the toxicity of the drug vehicle or excipient used in the formulation. Therefore, it is more common that anti-cancer drugs are delivered parenterally – either by IV infusion or injection. On top of reducing GI tract irritation, IV is the most effective way to deliver medication directly to the bloodstream, thereby increasing drug bioavailability. Nevertheless, achieving an acceptable bioavailability threshold is still hindered by poorly water-soluble APIs in the development pipeline.
Potential of next-generation parenteral excipients in oncology therapeutics
With current solubility-enhancing solutions not fully meeting the demands of the pharmaceutical industry, novel polymeric excipients are becoming an attractive option for the development of oncology therapeutics, as well as drugs for a variety of other diseases. Although the incorporation of novel excipients is rising in new drug formulations – especially in the search for effective anti-cancer agents – there is still hesitation from some developers and formulators.
With drug regulators tolerating a much higher risk-to-benefit ratio in the oncology space, suboptimal drug formulations have found it easier to make their way to market. However, it is this higher risk-to-benefit ratio and acceptance that has meant many more oncology formulators are open to the idea of leveraging novel excipients in their drug development projects. This will allow for a safer approach to drug formulation and the creation of therapeutics that are better tolerated by patients.
Apisolex™ polymer, developed by Lubrizol Life Science Health (LLS Health), is a versatile, efficient, and safe technology to help overcome the shortcomings of existing solubility enhancement techniques. [1][2][3][CFR4][CFR5][GJ6]Apisolex excipient is a biodegradable, biocompatible, amphiphilic block copolymer that utilizes micellar technology. Although polymeric micelles are not directly involved in API solubilization, their encapsulation mechanism is a commercially proven way to create stable nanoparticles that can help increase API solubility.
Improving solubility
API particle size reduction is an effective way to combat solubility issues by creating nanoparticles of crystalline API that can be used for virtually any route of administration. Polymeric micelles are another proven method to create stable nanoparticles. Apisolex polymer utilizes micellar technology to encapsulate molecules of hydrophobic API, enhancing API solubility by up to 50,000-fold with minimal API loss and more than 90% API recovery.
Table 1: Examples of polymeric micelle-based drug products in clinical trials or approved. Adapted from Hwang, D. et al (2020)4
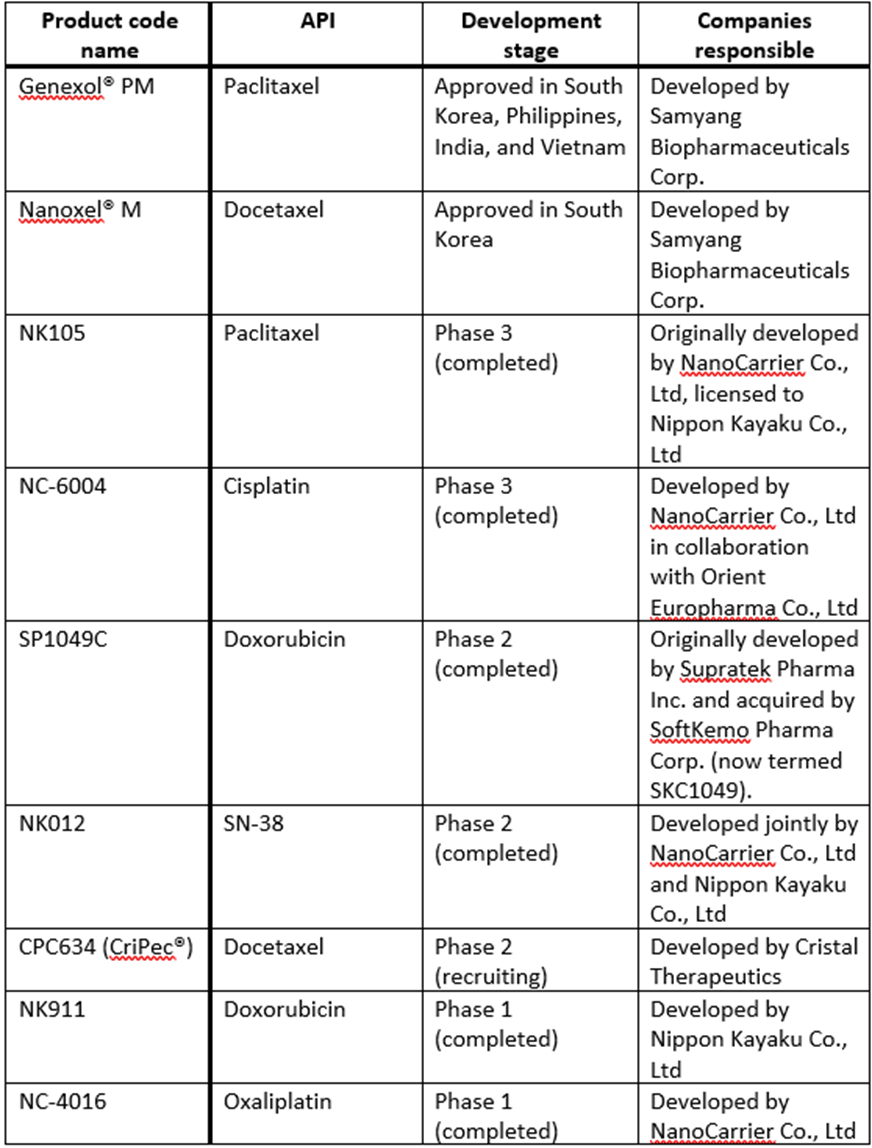
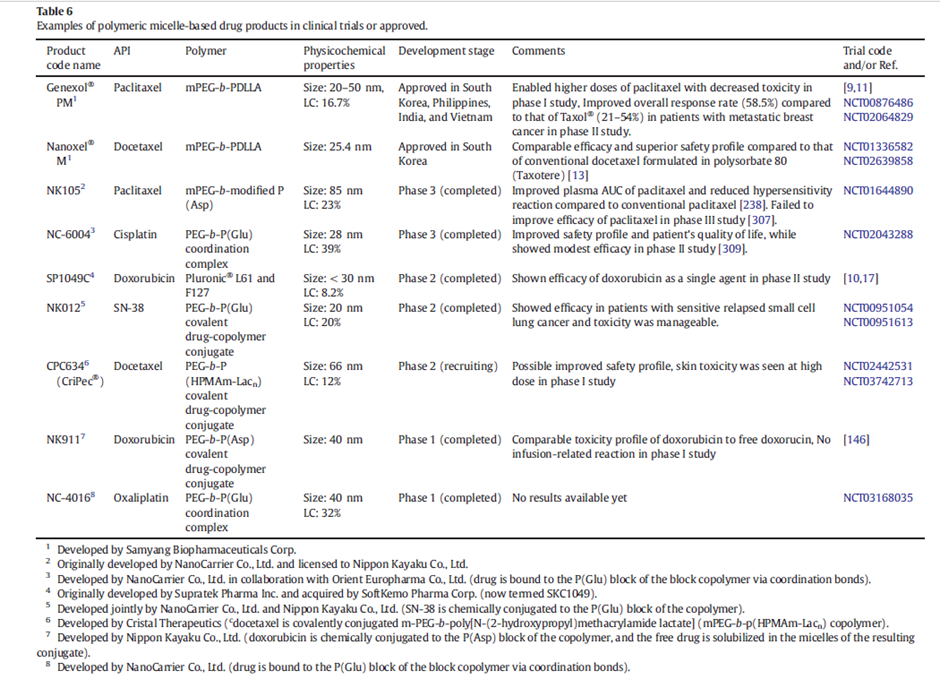
Enabling higher drug loading
Traditional inclusion complexing agents used in formulations only achieve API to solubilizer ratios of 1:100. However, Apisolex excipient can achieve an API to solubilizer ratio as high as 40:100. This greatly reduces the amount of polymeric excipient used in the final dosage form, allowing for an increased MTD or reduction in the overall volume of drug administered.
Improving safety
Compared to other water-soluble polymers like PEG, Apisolex polymer has a better safety and toxicity profile. Generated from safe amino acid-based building blocks, this amphiphilic copolymer incorporates a hydrophilic poly(sarcosine) block and a second drug-encapsulating block comprised of a mixture of hydrophobic D- and L- poly (amino acids). As such, Apisolex polymer exhibits inherently low toxicity.
Simplifying commercialization
One of the barriers that drug developers and formulators face when looking to improve the solubility of hydrophobic APIs is the use of complicated formulation techniques. However, some novel excipients – such as Apisolex polymer – employ simple, scalable techniques. This allows developers to progress from feasibility studies quickly and confidently, to GMP, and right on through to commercialization by leveraging processes they are familiar with. Additionally, novel excipients provide a level of IP protection that is not afforded by traditional excipients. They also allow for the reformulation of previously approved drugs via the FDA’s 505(b)(2) pathway. This provides formulators with the chance to expedite existing APIs to market and help create better life-changing therapeutics for patients in need.
Enhancing stability
The Apisolex polymer itself possesses a great stability profile. This allows for the creation of a stable lyophilized drug product or, when formulating a stable API, allows for a stable solution-based drug product. The lyophilized form of a drug created with Apisolex technology can be reconstituted in saline in less than 30 seconds.
Old drug new tricks: putting Apisolex polymer to the test with paclitaxel
Traditionally, Cremophor-ethanol (Kolliphor® EL) vehicles have been used to improve the solubility of parenteral oncology drugs, such as paclitaxel. However, Cremophor EL is not an inert drug vehicle. It has been known to exert a range of biological effects, such as hypotension, and has even been associated with anaphylaxis in some cases. (5)
In fact, patients who receive Cremophor EL-containing treatments often must be pre-dosed with steroids or antihistamines to reduce the adverse side effects of the vehicle. This example clearly demonstrates how dosage form and drug vehicle both limit the MTD of chemotherapy drugs.
By leveraging excipients with more attractive safety profiles, such as Apisolex polymer, the instance of infusion-related side effects can be greatly reduced, and it can also eliminate the need for premedication to abate adverse reactions. As such, the safety and toxicity of Apisolex excipient was evaluated in a study to improve the solubility of paclitaxel.
Results showed the Apisolex/paclitaxel formulation was well tolerated in test animals, demonstrating equivalent activity to paclitaxel on its own in terms of in vitro cytotoxicity, and in vivo tolerability. The lyophilized drug product was reconstituted in less than 30 seconds and the process was shown to be more than 90% efficient, with small particle size and narrow size distribution obtained. The Apisolex/paclitaxel formulation has further demonstrated more than 36 months’ stability under ambient conditions to date, with no change in physicochemical properties.
Time to try new approaches
For drug formulators, solubility and drug loading in parenteral dosage forms continue to be huge challenges. Furthermore, it is slowing the development of much-needed drug products, especially in the oncology market. Within the sector, it’s clear that the current solutions and technologies offered to enhance drug solubility are not meeting both formulator and patient needs. Safe, efficient, solubility-enabling technologies – such as Apisolex polymer – present a powerful solution for the most challenging parenteral projects and benefit both drug developers and patients. By improving acceptance of the use of novel excipients in parenteral formulations, formulators can continue to utilize simple and preferred formulation techniques to create better, life-changing therapeutics required in the market.
Request a sample of Apisolex excipient here
References
(1) Vo CL, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3 Pt B):799-813. doi:10.1016/j.ejpb.2013.09.007 <https://pubmed.ncbi.nlm.nih.gov/24056053/>
(2) Van Arnum P. Tracking Parenteral Drugs in New Drug Approvals. DCAT Value Chain Insights. Updated February 12, 2020. Accessed January 2023. <https://www.dcatvci.org/features/tracking-parenteral-drugs-in-new-drug-approvals/>
(3) Dupont P. Snapshot of the Parenteral Drug Delivery Market. Pharmaceutical International, Inc. Accessed January 2023 <https://www.pharm-int.com/2022/06/01/snapshot-of-the-parenteral-drug-delivery-market/>
(4) Hwang, D., Ramsey, J. D. & Kabanov, A. V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv Drug Deliv Rev. 2020; 156: 80–118. doi:10.1016/j.addr.2020.09.009 <https://www.sciencedirect.com/science/article/abs/pii/S0169409X20301332>
(5) Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/s0959-8049(01)00171-x <https://pubmed.ncbi.nlm.nih.gov/11527683/>


